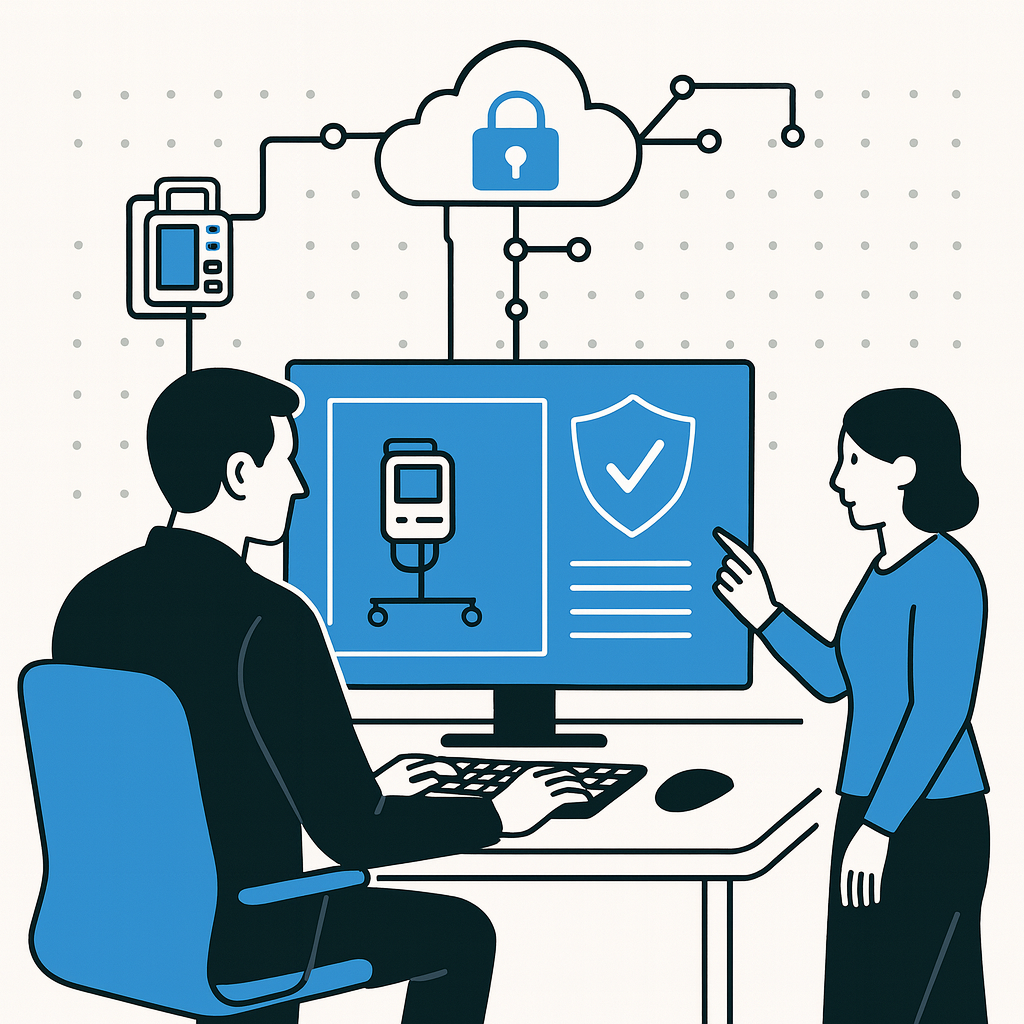
In our previous post, “Why TARA Matters in Medical Devices,” we explored how the Threat Analysis and Risk Assessment (TARA) framework revolutionized automotive safety — and why the healthcare sector stands to gain from adopting a similar, structured approach.
Today, we’ll go a step further and show, through a practical scenario, what happens when medical device security is left to chance — and how a Medical Device Cybersecurity Checklist can transform the outcome.
A Real-World Scenario: When Medical Device Security Fails
Imagine this:
A hospital relies on connected infusion pumps to deliver critical medication to patients. These devices communicate over the hospital’s Wi-Fi network, automatically sending treatment data into the EHR system. Everything operates seamlessly — until a security researcher discovers that the pump’s internal software can be accessed through its service port.
Suddenly, the risks become very real:
- A malicious actor could tamper with the pump’s dosage settings.
- Patient data could be exposed during wireless transmission.
- A cyber incident could interrupt life-saving therapy at the bedside.
Within moments, the hospital’s IT and clinical engineering teams are asking urgent questions:
- How do we know if our devices are safe from tampering?
- Are we meeting FDA and HIPAA cybersecurity expectations?
- What evidence could we provide if regulators demanded proof tomorrow?
Where TARA Makes the Difference
This is exactly where a structured TARA approach can turn chaos into control.
By systematically identifying assets, risks, and threat scenarios — not just for the device itself, but also its data flows, cloud connections, and update mechanisms — hidden vulnerabilities are uncovered early.
Each step of the process aligns with recognized frameworks, including:
- FDA Premarket Cybersecurity Guidance
- HIPAA Security Rule
- ISO 14971: Risk Management for Medical Devices
- IEC 62304: Medical Device Software Lifecycle Processes
The result? A comprehensive view of risks and mitigations, supported by a clear risk register, documented test results, and compliance-ready evidence.
With this foundation, hospitals and manufacturers no longer scramble to react after a breach — they proactively protect patients and maintain compliance.
The Benefits of Using a Medical Device Cybersecurity Checklist
Instead of responding under pressure, organizations gain:
- Confidence that devices remain safe for patient use.
- Assurance that regulatory requirements are already met.
- Consistency through a repeatable cybersecurity lifecycle.
- Preparedness for audits and FDA submissions.
- Resilience against evolving cyber threats.
A structured checklist based on TARA is more than documentation; it’s an operational advantage that integrates cybersecurity into every phase of the product lifecycle.
Introducing the Medical Device Cybersecurity Checklist
To make this process simpler, we’ve developed the Medical Device Cybersecurity Checklist — a practical, step-by-step guide that helps healthcare IT and clinical teams choose the right security measures for connected medical systems.
Built upon our proven TARA and risk-based methodology, the checklist ensures you don’t have to start from scratch. It enables you to:
- Identify critical assets and data flows.
- Map potential attack vectors.
- Prioritize mitigations based on likelihood and impact.
- Document evidence for FDA and HIPAA compliance.
- Maintain ongoing risk monitoring across the device lifecycle.
Frequently Asked Questions (FAQs)
1. What is the purpose of a Medical Device Cybersecurity Checklist?
It’s a structured guide that helps organizations identify vulnerabilities, document mitigations, and maintain compliance with standards like FDA Premarket Guidance and HIPAA.
2. How does TARA improve medical device security?
TARA (Threat Analysis and Risk Assessment) brings a systematic process to identify and prioritize cyber risks, ensuring that security is proactive, not reactive.
3. Do all medical devices need cybersecurity assessments?
Yes. Any device that connects to a network, transmits patient data, or can be remotely accessed must undergo a cybersecurity assessment to ensure safety and compliance.
4. How often should cybersecurity reviews be performed?
Cybersecurity reviews should be performed periodically — ideally at every software update or major configuration change — to account for emerging threats.
5. Can hospitals use the same checklist as manufacturers?
While both can use the same framework, hospitals often focus on operational security controls and incident response, whereas manufacturers focus on design-phase risk management.
6. What are the key standards for medical device cybersecurity?
The most relevant standards include FDA Premarket Guidance, ISO 14971, IEC 62304, and AAMI TIR57, all of which integrate risk-based cybersecurity principles.
Conclusion: Secure Devices, Confident Care
A Medical Device Cybersecurity Checklist isn’t just another compliance tool — it’s the foundation of patient trust and operational continuity.
By integrating TARA principles and aligning with industry standards, healthcare organizations can move from reactive fixes to proactive resilience.
The result?
- Fewer surprises,
- Stronger compliance posture, and
- Safer patient outcomes.
Start today — download your Medical Device Cybersecurity Checklist and take the first step toward a safer, more secure healthcare environment.
Take Action: Get A Free Medical Device Cybersecurity Checklist
Take the first step toward safer, compliant connected systems. Our checklist gives you a structured approach to TARA, helping you identify risks, document mitigations, and ensure audit-ready medical device security.
Stay Connected with Block Harbor
Keep up with the latest in vehicle cybersecurity through our specialized newsletters. Choose the option that best fits your interests and role.
Thank you for your submission!
Read More
Explore more automotive cybersecurity insights from our experts. Discover best practices, case studies, and emerging trends to strengthen your organization's security posture.
The state of automotive cybersecurity today and the forces that will define what comes next.
Announcement of Block Harbor’s selection for the Google AI Academy: American Infrastructure, highlighting our mission to secure AI-powered mobility systems like connected vehicles, drones, and robots through the VSEC cybersecurity platform.

Explore how VSOC and PSIRT work together to enhance cybersecurity in connected vehicles—balancing real-time monitoring with long-term vulnerability management.
Try Block Harbor Today
Start protecting your vehicles with the same platform the world’s best hackers and defenders use.


%201.svg)